A) I
B) II
C) III
D) IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is/are the product(s) of the following monochlorination? 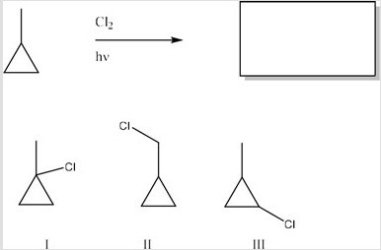
A) I
B) II
C) III
D) All of the choices are correct.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many monochlorination products can be formed from the reaction of (CH3) 3CH with Cl2 and hn?
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many monochlorination products can be formed (constitutional isomers only) from the reaction of (CH3) 2CHCH2CH3 with Cl2 and hn?
A) 2
B) 3
C) 4
D) 5
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the monomer used to make the following polymer. 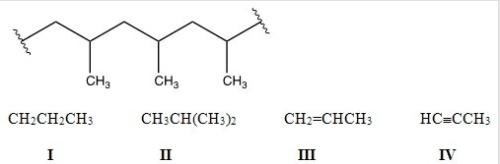
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the monomer used to make the following polymer. 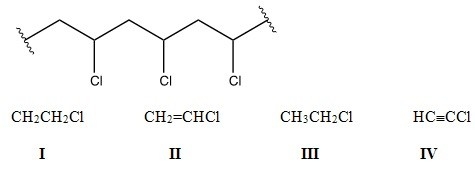
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A possible reaction of ethane with chlorine is shown below. ![A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].Determine DH for step [1]. Bond dissociation energies (kcal/mol) : A) -5 kcal/mol B) +58 kcal/mol C) -28 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7cd_25fe_a9af_c756cc3791cf_TB7662_00.jpg) This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].Determine DH for step [1].
This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].Determine DH for step [1]. ![A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].Determine DH for step [1]. Bond dissociation energies (kcal/mol) : A) -5 kcal/mol B) +58 kcal/mol C) -28 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7cd_25ff_a9af_ab3bba37a3c7_TB7662_00.jpg) Bond dissociation energies (kcal/mol) :
Bond dissociation energies (kcal/mol) : ![A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].Determine DH for step [1]. Bond dissociation energies (kcal/mol) : A) -5 kcal/mol B) +58 kcal/mol C) -28 kcal/mol D) None of the choices are correct.](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7cd_2600_a9af_c9f8ee2cd92c_TB7662_00.jpg)
A) -5 kcal/mol
B) +58 kcal/mol
C) -28 kcal/mol
D) None of the choices are correct.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the propagation steps in the chlorination of ethane is true?
A) Radical chlorination consists of two propagation steps.
B) The energy diagram for the propagation steps has three energy barriers.
C) The first of the propagation steps is rate-determining because its transition state is at lower energy.
D) The second of the propagation steps is rate-determining because its transition state is at higher energy.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is (are) true about free radical halogenation of alkanes?
A) The first of the chain-propagating steps is rate-determining.
B) The reaction proceeds by way of a flat sp2 hybridized free radical.
C) The chain-initiating step involves cleavage of a carbon-hydrogen bond to afford a carbon radical and a hydrogen atom.
D) Statements (The first of the chain-propagating steps is rate-determining) and (The reaction proceeds by way of a flat sp2 hybridized free radical) are both true.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the indicated hydrogens is most readily abstracted in a free radical halogenation reaction? 
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A possible reaction of ethane with chlorine is shown below. ![A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].The chain initiating step(s) is (are) ________. A) Only [1] B) Only [2] C) Only [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7cc_b0ca_a9af_a505cce78e37_TB7662_00.jpg) This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].The chain initiating step(s) is (are) ________.
This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].The chain initiating step(s) is (are) ________. ![A possible reaction of ethane with chlorine is shown below. This reaction could conceivably occur by the following chain mechanisms [1],[2],and [3].The chain initiating step(s) is (are) ________. A) Only [1] B) Only [2] C) Only [3] D) Only [1] and [2]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f7cc_b0cb_a9af_5dcb4b24a603_TB7662_00.jpg)
A) Only [1]
B) Only [2]
C) Only [3]
D) Only [1] and [2]
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 51 of 51
Related Exams