B) False
Correct Answer

verified
Correct Answer
verified
True/False
Bond dissociation energies are always positive numbers.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Heat is absorbed in an ________ reaction.
Correct Answer

verified
Correct Answer
verified
True/False
The equilibrium constant expression for the reversible reaction: CHCl3(g)+ 3 HCl(g)  CH4(g)+ 3 Cl2(g)is
CH4(g)+ 3 Cl2(g)is 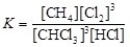 .
.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Bond breaking is endothermic.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The ΔH for the reaction depicted by the energy diagram below is ________. 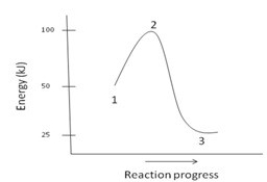
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reversible reaction at equilibrium: N2(g) + O2(g) 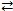 2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
2 NO(g) . What is the effect of removing some N2(g) from the equilibrium system?
A) The concentration of O2(g) increases
B) The concentration of O2(g) decreases
C) The concentration of NO(g) increases
D) The equilibrium system shifts to the right
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equilibrium constant with a value of 8.0 × 106 indicates that at equilibrium ________.
A) the reactants are favored
B) the products are favored
C) approximately equal concentrations of reactants and products are present
D) there are more reactants present than products
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
When reactants can come together and form products,and products can come together to re-form reactants,the reaction is called a ________ reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is ALWAYS necessary for a chemical reaction to occur between two reactants,A and B?
A) Equal amounts of A and B must be present
B) A and B must be present in the same physical state
C) The reaction must be carried out at a temperature higher than room temperature
D) A and B must collide with the proper orientation and with a certain minimum amount of energy
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction,3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g) ,where ΔH = -137 kJ. How many kilojoules are released when 92.3 g of NO2 reacts?
A) 2.01 kJ are released
B) 2.75 × 102 kJ are released
C) 91.6 kJ are released
D) 1.26 × 104 kJ are released
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the pressure of a reaction at equilibrium decreases,in which direction does the equilibrium shift?
A) The equilibrium shifts in the direction that increases the number of moles of gas.
B) The equilibrium shifts in the direction that decreases the number of moles of gas.
C) The equilibrium does not shift.
D) The equilibrium shifts always shifts to the right.
E) The equilibrium shifts always shifts to the left.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reversible reaction: PCl3(g) + Cl2(g)  PCl5(g) ,has K = 0.5. Based on the molecular art shown below,what can be inferred about the reaction conditions?
PCl5(g) ,has K = 0.5. Based on the molecular art shown below,what can be inferred about the reaction conditions? 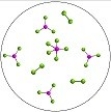
A) The reaction has not yet reached equilibrium.
B) The reaction mixture is at equilibrium.
C) The reaction will shift to the left to reach equilibrium.
D) The forward reaction is favorable.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Once equilibrium is reached in a chemical reaction,reactants stop forming products.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Le Châtelier's principle is a general rule used to explain the effect of a change in reaction conditions on equilibrium.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Changes in potential energy occur in chemical reactions.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
When the equilibrium constant for a reaction is much less than one (K < 1),the concentration of the products is larger than the concentration of the reactants.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One step in the metabolism of glucose is depicted below. Which statement best describes how the equilibrium system would respond if the amount of dihydroxyacetone phosphate is decreased? 
A) The system would shift to the left,consuming more dihydroxyacetone phosphate.
B) The system would shift to the right,producing more dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
C) The system would shift to the left,producing more fructose 1,6-bisphosphate.
D) The system would shift to the right,consuming some glyceraldehyde 3-phosphate and producing more dihydroxyacetone phosphate.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
In order to cause an endothermic equilibrium reaction to shift in a direction to form more products,the reaction must be heated.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction requires 31.39 kJ. How many kilocalories does this correspond to?
A) 7,502 kcal
B) 7.502 kcal
C) 131.3 kcal
D) 0.1313 kcal
E) 31.39 kcal
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 102
Related Exams