A) experiences a loss of electrons.
B) takes on oxygen atoms.
C) experiences a gain of electrons.
D) becomes a charged species.
E) gives up hydrogen atoms.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 85.0 g of 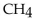 are mixed with 160 g of
are mixed with 160 g of 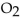 the limiting reactant is
the limiting reactant is
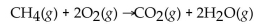
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of  are in 15.0 g of
are in 15.0 g of 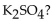
A) 0.119 moles
B) 0.111 moles
C) 0.0861 moles
D) 0.172 moles
E) 2.61 x 103 moles
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Barium chloride and sodium sulfate react according to the following equation. 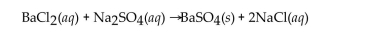 Answer the question(s) that follow about this reaction.
-How many moles of barium sulfate are produced from 0.100 mole of barium chloride?
Answer the question(s) that follow about this reaction.
-How many moles of barium sulfate are produced from 0.100 mole of barium chloride?
A) 0.100 mole
B) 1.00 mole
C) 2.00 moles
D) 0.0100 mole
E) 0.200 mole
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the question(s) that follow, consider the following equation. 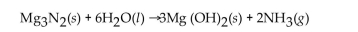 -When 2 moles of
-When 2 moles of 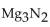 are allowed to react, how many moles of
are allowed to react, how many moles of 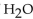 also react?
also react?
A) 6 moles
B) 4 moles
C) 1 mole
D) 12 moles
E) 8 moles
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1.25 moles of 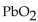 have a mass of
have a mass of
A) 279 g.
B) 299 g.
C) 239 g.
D) 191 g.
E) 178 g.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 60.0 g of 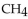 reacts with excess
reacts with excess 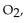 the actual yield of
the actual yield of 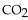 is 112 g. What is the percent yield?
is 112 g. What is the percent yield?
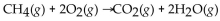
A) 67.9 %
B) 46.4 %
C) 187 %
D) 53.6 %
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of sodium phosphate, 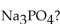
A) 308 g
B) 119 g
C) 164 g
D) 226 g
E) 354 g
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the mass of 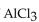 that is produced when 10.0 grams of
that is produced when 10.0 grams of 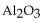 react with 10.0 g of HCl according to the following equation.
react with 10.0 g of HCl according to the following equation.
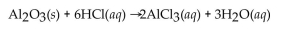
A) 10.0 g
B) 6.10 g
C) 16.2 g
D) 12.2 g
E) 20.0 g
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the classification for this balanced reaction? 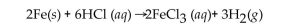
A) double replacement
B) decomposition
C) combination
D) combustion
E) single replacement
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the question(s) that follow, consider the following equation. 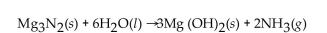 -How many grams of
-How many grams of  are needed to produce 150 g of
are needed to produce 150 g of 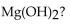
A) 23 g
B) 18 g
C) 46 g
D) 93 g
E) 130 g
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the molar mass of NaBr?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of neon are present in 1.30 moles of neon?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
4.00 moles of sodium have a mass of
A) 44.0 g.
B) 23.0 g.
C) 11.0 g.
D) 4.60 g.
E) 92.0 g.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 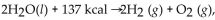 how many kcal are needed to react
how many kcal are needed to react 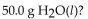
A) 274 kcal
B) 190 kcal
C) 760. kcal
D) 137 kcal
E) 380 kcal
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction, when the equation is correctly balanced, what is the correct coefficient for aluminum chloride? 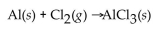
A) 1
B) 2
C) 3
D) 4
E) 5
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 96 of 96
Related Exams