A) two single bonds, the sum of absolute values of formal charges = 3, and no resonance hybrids.
B) one single and one double bond, sum of absolute values of formal charges = 5, and two contributing resonance hybrids.
C) one single and one double bond, sum of absolute values of formal charges = 3, and two contributing resonance hybrids.
D) two double bonds, sum of absolute values of formal charges = 1, and no resonance hybrids.
E) one single and one double bond, sum of absolute values of formal charges = 1, and two contributing resonance hybrids.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
When the Sn2+ ion is formed, the electrons are removed from which set of orbital(s)?
Correct Answer

verified
5p
Correct Answer
verified
Short Answer
Which of these species, Fe3+, P3−, B−, Ca2+, has the most valence electrons?
Correct Answer

verified
Correct Answer
verified
Short Answer
The total number of bonds in the thiocyanate ion, SCN−.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium forms a monatomic ion that has the electron configuration of a noble gas. What is the electron configuration of that noble gas?
A) 1s2
B) 1s2 2p6
C) 1s2 2s2 2p6
D) 1s2 2s2 2p6 3s2
E) 1s2 2s2 2p6 3s2 3p6
G) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
In the family of compounds called aldehydes, the carbon in C=O group is directly attached to another ________ atom and a ________ atom.
Correct Answer

verified
Correct Answer
verified
Short Answer
Which of these ions shown, Fe3+, P3− , B−, Ca2+, has the same number of valence electrons as a germanium atom?
Correct Answer

verified
B−
Correct Answer
verified
Short Answer
The Lewis symbol for the carbon atom shows ________ valence shell electrons. The number of covalent bonds that carbon normally forms to complete its valence shell and obey the octet rule is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond is the most polar?Hint: Consider the electronegativity values of each element.
A) B-C
B) C-N
C) C-O
D) Si-O
E) C-C
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the "best"Lewis structure after applying formal charge considerations, how many non-bonding valence electrons are around the nitrogen atom in the HCN molecule?
A) 0
B) 1
C) 2
D) 4
E) 6
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
There are three resonance structures for the hydrogen carbonate ion. Hint: Draw the Lewis structure and look at the double bonds. How could they be re-arranged?
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
The total number of non-bonding electrons in molecular oxygen is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Draw the most favorable Lewis structure for the NCO molecule. The formal charge on the oxygen molecule is ________. Hint: Consider double and triple bonds for your structure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that the first ionization energy of cesium is +376 kJ/mol and the electron affinity of bromine is -325 kJ/mol, calculate E for the reaction, Cs(g) + Br(g) s Cs+(g) + Br?(g) .
A) +376 kJ/mol
B) +701 kJ/mol
C) +51 kJ/mol
D) -701 kJ/mol
E) -51 kJ/mol
G) D) and E)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following solids would have the highest melting point?
A) LiF
B) NaCl
C) AlCl3
D) Al2O3
E) CaCl2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The formal charge on the most stable Lewis structure for the central oxygen in ozone, O3, is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Draw the Lewis structure for SOCl2. Considering formal charges, there will be ________ bonds and ________ resonance structures in the molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solids is likely to have the largest exothermic lattice energy?
A) LiF
B) NaCl
C) AlCl3
D) Al2O3
E) CaCl2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A student drew four possible Lewis structures for HBrO4 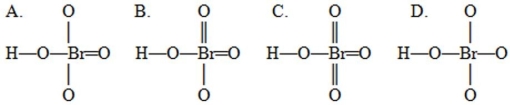 Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
Complete these Lewis structures presented above by filling in the remaining valence electrons that are not in the bonds. Based on these structures, the preferred structure would be the structure shown as ________ in which the sum of the absolute values of the formal charges on all the atoms is ________. Hint: When determining remaining valence electrons remember to account for the electrons already present in the bonds.
A) A, 4
B) B, 2
C) C, 0
D) D, 6
E) D, 0
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ionic solid is likely to have the least exothermic lattice energy?
A) KCl
B) NaCl
C) LiCl
D) CsCl
E) RbBr
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 167
Related Exams