A) 2.1 × 10-10 M
B) 9.7 × 10-10 M
C) 4.7 × 10-5 M
D) 3.8 × 10-5 M
E) 6.3 × 10-6 M
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of an aqueous solution at 25.0°C is 10.66.What is the molarity of 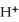 in this solution?
in this solution?
A) 2.2 × 10-11
B) 4.6 × 10-4
C) 3.3
D) 1.1 × 10-13
E) 4.6 × 1010
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is amphoteric?
A) SO42-
B) HF
C) NH4+
D) HPO42-
E) LiI
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a strong base?
A) Br-
B) NH2CH2CH3
C) CH3OH
D) NO3⁻
E) NaOH
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of a 0.62 M NH4NO3 solution at 25°C.The Kb for NH3 is 1.76 × 10-5.
A) 2.48
B) 9.27
C) 11.52
D) 4.73
E) 9.45
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a strong acid?
A) H COOH
B) HF
C) HI
D) NH4+
E) H2S
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the Ka of an acid whose 0.294 M solution has a pH of 2.80.
A) 1.2 × 10-5
B) 8.5 × 10-6
C) 2.7
D) 4.9 × 10-7
E) 5.4 × 10-3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List an ingredient that is in antacid.
A) Al(OH) 3
B) Mg(OH) 2
C) CaCO3
D) all of the above
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the strongest acid.
A) HFO4
B) HFO3
C) HFO2
D) HFO
E) Not enough information is given.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the weak monoprotic acid.
A) H2CO3
B) NaOH
C) HCOOH
D) HI
E) KBr
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the characteristics of a strong acid.
A) ionizes completely in aqueous solutions
B) has equilibrium far to the right
C) has a polar bond
D) has a weaker bond to hydrogen
E) all of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
What is the difference between a strong and weak acid?
Correct Answer

verified
A strong acid ionizes/dissocia...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine the [OH⁻] concentration in a 0.344 M Ba(OH) 2 solution.
A) 0.688 M
B) 0.344 M
C) 0.463
D) 0.334
E) 13.666
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.40 M H2Se solution that has the stepwise dissociation constants Ka1 = 1.3 × 10-4 and Ka2 = 1.0 × 10-11?
A) 2.14
B) 3.89
C) 4.28
D) 5.57
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of a 0.00598 M HClO4 solution.
A) 11.777
B) 6.434
C) 7.566
D) 2.223
E) 3.558
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following salts,when 1 mole is dissolved in water,produces the solution with a pH closest to 7.00?
A) NH4ClO4
B) Na2O
C) NaHSO4
D) NaF
E) NaOH
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a Br∅nsted-Lowry acid?
A) NH4+
B) CBr4
C) PH2-
D) NH3
E) F2
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The stomach excretes ________ to kill microorganisms and to activate enzymes that break down food.
A) H2CO3
B) HBr
C) KHCO3
D) HCl
E) HNO3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of a 0.232 M Mg(OH) 2 solution at 25°C.
A) 12.73
B) 13.37
C) 13.67
D) 0.333
E) 0.634
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.0040 M Sr(OH) 2 solution?
A) 2.10
B) 2.40
C) 11.60
D) 11.90
E) 12.00
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 160
Related Exams