A) 1. KOtBu; 2. 1-bromopropane
B) 1. NaNH2; 2. 1-bromopropane
C) 1. NaNH2; 2. 2-bromopropane
D) 1. O3; 2. DMS
E) 1. O3; 2. 1-bromopropane
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Perform a retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the alkyne (C) . 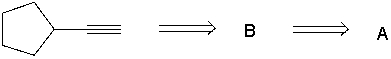
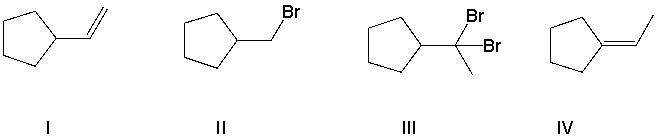
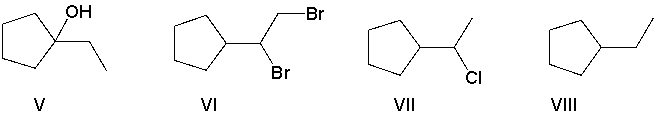
A) B = I and A = VI
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = VI and A = VIII
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Devise a synthetic route to convert 5-methyl-1-hexene into 5-methylhexanal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best reagents for the reaction below. 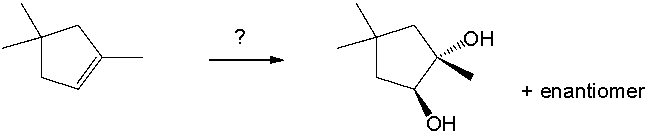
A) 1. OsO4; 2. NaHSO3, H2O
B) 1. Hg(OAc) 2, H2O; 2. NaBH4
C) 1. RCO3H; 2. H3O+
D) H2SO4, H2O
E) 1. O3; 2. DMS
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sequences of reagents will move the alcohol functional group from the tertiary position of 1-methylcyclohexanol to a secondary position?
A) 1. KOtBu; 2. Hg(OAc) 2, H2O; 3. NaBH4
B) 1. TsCl, pyr; 2. KOtBu; 3. BH3-THF; 4. H2O2, NaOH
C) 1. H2SO4, heat; 2. BH3∙THF; 3. H2O2, NaOH
D) 1. TsCl, pyr; 2. NaOH; 3. BH3∙THF; 4. H2O2, NaOH
E) C and D will both work
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best reagents for the reaction below. 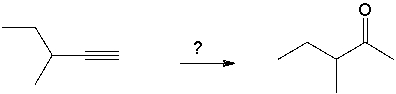
A) 1. OsO4; 2. NaHSO3, H2O
B) H2SO4, H2O, HgSO4
C) H2, Pt
D) 1. 9-BBN; 2. H2O2, NaOH
E) 1. O3; 2. DMS
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 106 of 106
Related Exams