A) 28.01
B) 384.4
C) 32.00
D) 44.01
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms are in 1.50 moles of fluorine gas?
A) 6.022 × 1023
B) 9.03 × 1023
C) 18.98
D) 1.81 × 1024
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound composed of only carbon and hydrogen is 25.2% hydrogen by mass.The empirical formula for this compound is:
A) CH 0.25
B) C3H
C) CH4
D) C2H8
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vitamin C is known chemically by the name ascorbic acid.Determine the empirical formula of ascorbic acid if it is composed of 40.92% carbon,4.58% hydrogen,and 54.50% oxygen.
A) CHO
B) CH2O
C) C2H3O2
D) C3H4O3
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you have 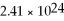 Atoms of copper,how many moles of copper do you have?
Atoms of copper,how many moles of copper do you have?
A) 0.250
B) 4.00
C) 0.600
D) 3.00
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 42.7 gram sample of potassium nitrate contains how many grams of potassium?
A) 39.1
B) 16.5
C) 21.4
D) 8.54
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of iron are contained in 1.75 kg of iron?
A) 3.13 × 10-2
B) 3.13 × 10-4
C) 31.3
D) 3.13 × 104
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
A molecule that has an empirical formula of HO and a molar mass of 34.02 gram must have a molecular formula of H2O2.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The empirical formula mass must be 25.0 if the molecular formula mass is 250 and n = 5.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have 10.0 g each of Na,C,Pb,Cu and Ne.Which contains the smallest number of moles?
A) Na
B) C
C) Pb
D) Cu
E) Ne
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of aluminum sulfate?
A) 123.0 g/mol
B) 278.0 g/mol
C) 306.2 g/mol
D) 315.2 g/mol
E) 342.2 g/mol
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have 10.0 g each of Na,C,Pb,Cu and Ne.Which contains the largest number of moles?
A) Na
B) C
C) Pb
D) Cu
E) Ne
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are in 35.0 grams of hydrogen gas?
A) 4.25 × 1025
B) 2.09 × 1025
C) 2.12 × 1025
D) 1.05 × 1025
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The correct formula for calculating mass percent of X in compound XY is: 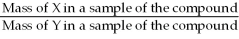 = Mass % X
= Mass % X
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of potassium sulfate contains:
A) 4 moles of oxygen.
B) 2 moles of sulfur.
C) 1 mole of potassium.
D) 3 moles of potassium.
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a sample of carbon dioxide contains 3.8 moles of oxygen atoms,how many moles of carbon dioxide are in the sample?
A) 1.9
B) 3.8
C) 7.6
D) 11.4
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
One mole of I2 has more atoms in it than one mole of Na.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct value for Avogadro's number?
A) 6.022 × 1023
B) 6.022 × 1033
C) 6.023 × 1022
D) 6.022 × 102.3
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains 9.02 × 1023 atoms?
A) 4.00 g H2
B) 9.00 g H2O
C) 28.0 g N2
D) 32.0 g O2
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The molecular formula is equal to the empirical formula multiplied by a whole number integer.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 118
Related Exams