A) The system would shift to the left, consuming more dihydroxyacetone phosphate.
B) The system would shift to the right, producing more dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
C) The system would shift to the left, producing more fructose 1, 6-bisphosphate.
D) The system would shift to the right, consuming some glyceraldehyde 3-phosphate and producing more dihydroxyacetone phosphate.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Increasing the temperature of a reaction mixture usually results in a decrease in the reaction rate.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Le Châtelier's principle is a general rule used to explain the effect of a change in reaction conditions on equilibrium.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a reasonable assumption about the chemical reaction whose energy diagram is depicted below? 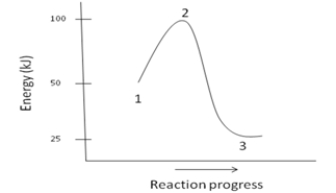
A) The activation energy for the reaction is 100kJ.
B) The reaction is exothermic.
C) H= -25kJ
D) The reaction is favorable.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning the reversible reaction 2 NO2(g)  N2O4(g) is true?
N2O4(g) is true?
A) NO2 is the product of the forward reaction.
B) The reverse reaction produces N2O4.
C) At the start of the reaction, the forward and reverse reaction rates are equal.
D) As the forward reaction progresses and more N2O4 is formed, the reverse reaction rate increases.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction, C2H4(g) + H2(g) C2H6(g) , where H = -137 kJ. How many kilojoules are released when 55.3 g of C2H4 reacts?
A) 137 kJ are released
B) 270. kJ are released
C) 1.13 × 103 kJ are released
D) 7.58 × 103 kJ are released
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: N2(g) + O2(g) ![Consider the reaction: N<sub>2</sub>(g) + O<sub>2</sub>(g) 2 NO(g) . If [N<sub>2</sub>] = 0.520 M, [O<sub>2</sub>] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K? A) 3.60 B) 0.278 C) 377 D) 0.00265](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e50_9547_8585aa67b6d2_TB5866_11.jpg) 2 NO(g) . If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?
2 NO(g) . If [N2] = 0.520 M, [O2] = 0.0662 M, and [NO] = 0.00956 at equilibrium, what is the value of K?
A) 3.60
B) 0.278
C) 377
D) 0.00265
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
Kinetic energy is the energy associated with movement; potential energy is the energy inherent in an object due to its position or composition.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Once equilibrium is reached in a chemical reaction, reactants stop forming products.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: PCl3(g) + Cl2(g) ![Consider the reaction: PCl<sub>3</sub>(g) + Cl<sub>2</sub>(g) PCl<sub>5</sub>(g) . If [PCl<sub>3</sub>] = 0.78 M, [Cl<sub>2</sub>] = 0.44 M, and [PCl<sub>5</sub>] = 0.88 at equilibrium, what is the value of K? A) 0.39 B) 1.4 C) 2.6 D) 0.72](https://d2lvgg3v3hfg70.cloudfront.net/TB5866/11eaaeeb_de42_0e4f_9547_854b454e379f_TB5866_11.jpg) PCl5(g) . If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?
PCl5(g) . If [PCl3] = 0.78 M, [Cl2] = 0.44 M, and [PCl5] = 0.88 at equilibrium, what is the value of K?
A) 0.39
B) 1.4
C) 2.6
D) 0.72
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equilibrium constant with a value of 8.0 × 106 indicates that at equilibrium
A) the reactants are favored.
B) the products are favored.
C) approximately equal concentrations of reactants and products are present.
D) there are more reactants present than products.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Exothermic reactions involve the formation of products having lower energy than the reactants.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Bond dissociation energies are always positive numbers.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
A reversible reaction in which K = 9.65 × 10-14 contains a negligible amount of reactants at equilibrium.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
When heat is added to an exothermic equilibrium reaction, the reaction shifts to form more ____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Walking at a brisk pace burns off about 280 Cal/h. How long would you have to walk to burn off the Calories obtained from eating a candy bar that contained 3 g of protein, 12 g of fat, and 28 g of carbohydrates?
A) 55 minutes
B) 230 minutes
C) 50 minutes
D) 210 minutes
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The H for the reaction depicted by the energy diagram below is ____. 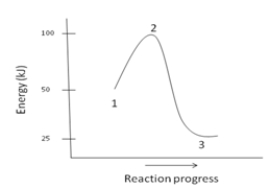
Correct Answer

verified
Correct Answer
verified
Short Answer
In the energy diagram shown below, C labels the _____. 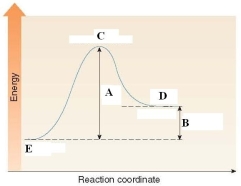
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term correctly describes a reaction in which the energy of the products is higher than the energy of the reactants?
A) oxidation-reduction
B) endothermic
C) exothermic
D) combustion
F) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The expression for the equilibrium constant, K, for the general reaction: a A + b B 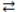 c C + d D is
c C + d D is 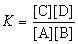 .
.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 88
Related Exams