A) PCl5
B) OCl6
C) SCl6
D) All of these have stable Lewis structures.
E) None of these has a stable Lewis structure.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for IF6+.
A) tetrahedral
B) pyramidal
C) octahedral
D) square planar
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Estimate the bond energy of the N2 molecule. 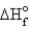 for NH3 = -46.0 kJ/mol
N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
for NH3 = -46.0 kJ/mol
N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the molecule with the strongest bond.
A) HF
B) NH3
C) H2O
D) CH4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecules. I. BF3 II. CHBr3 (C is the central atom.) III. Br2 IV. XeCl2 V. CO VI. SF4 Select the molecule(s) that fit the given statement.These molecules violate the octet rule.
A) III, V, VI
B) I, II, IV, VI
C) I, IV, VI
D) I, II, IV
E) I, III, IV, VI
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion violates the octet rule?
A) CH4
B) I3-
C) NO3-
D) SO3
E) CO2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the molecule with the strongest bond.
A) HBr
B) HCl
C) HF
D) HI
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains a double bond?
A) CO2
B) H2O
C) NH3
D) all
E) none
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
How many lone pairs of electrons are around the central atom?
Correct Answer

verified
one lone p...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following has the Lewis structure most like that of CO32-?
A) SO32-
B) CO2
C) NO3-
D) NO2
E) O3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the statement that best describes the PbCl4 molecule in the gas phase.
A) The molecule is polar.
B) The bonds are nonpolar.
C) The molecule is polar with bond angles of about 109°.
D) The bond angles are all about 109°.
E) The molecule has a dipole moment.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a dipole moment?
A) SCl6
B) CO2
C) OF2
D) BH3
E) None of these has a dipole moment.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on electronegativities, which of the following would you expect to be most ionic?
A) N2
B) CO2
C) CF4
D) CH4
E) CaF2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Refer to the SeF4 molecule. -What is the electron arrangement around the central atom?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for NH3.
A) linear
B) tetrahedral
C) pyramidal
D) bent
E) none of these
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the compound crotonaldehyde, whose skeleton is 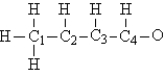 Which carbon in this molecule has tetrahedral bonding?
Which carbon in this molecule has tetrahedral bonding?
A) 2
B) 3
C) 4
D) 1
E) All have tetrahedral bonding.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures best describes BF3?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules and ions has a lone pair of electrons on the central atom?
A) PCl5
B) XeO4
C) BeCl2
D) CH3+
E) CH3-
G) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
For each of the following compounds: A) Give the shape of the molecule. B) Indicate the polarity of the molecule. -OCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the Lewis structure for elemental nitrogen, there is(are)
A) a triple bond between the nitrogens.
B) three unpaired electrons.
C) a double bond between the nitrogens.
D) a single bond between the nitrogens.
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 136
Related Exams